Reformatsky Reaction
Reformatsky Reaction
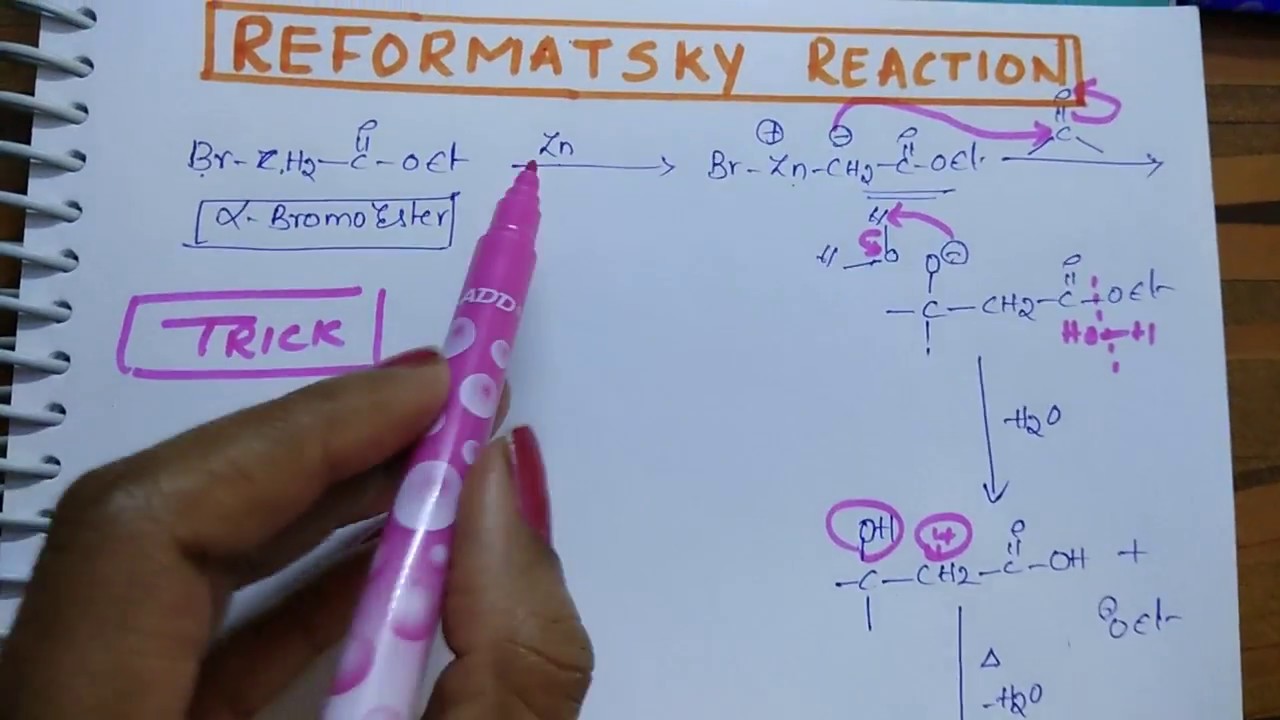
Condensation reaction of carbony compound with α-haloester in prescence of zinc metal is known as Reformatsky reaction”. Inert solvent like diehyl ether or tetrahydrofuran(THF) are often used as a solvent for this reaction, sometimes benzene or ether or benzene ether mixture may also be used.
It is an addition reaction in which an organozinc reagent is used instead of Grignard’s reagent, to attack the carbonyl group of an aldehyde or ketone. Since the organozinc is less reactive than Grignard’s reagent, a nucleophilic addition to ester group does not take place. The organozinc reagent is prepared by the reaction of an α-bromoester with zinc metal.
Course Material
- Kinetic control vs thermodynamic control
- Selectivity (stereo, regio, chemo)
- Active methylene compounds
- Aldol Condensation
- Claisen condensation
- Cross Claisen Condensation
- Reformatsky Reaction
- Stobbes Condensation
- Darzen condensation
- Perkin condensation
- Wittig Reaction
- Nucleophilic Substitution Reactions
- SN Reactions I
- SN Reactions II
- SN Reactions III
- Nucleophilic Substitution Prime Reactions
- Neighbouring group participation
- Aromatic Electrophilic Substitution Reactions
- NAS Elimination Addition
- NAS Addition Elimination
- Aromatic Nucleophilic Reactions (diazonium salts)
- Elimination Reactions
- Reactive intermediates
- Chapters 23
- Department Chemistry
- Teacher
Dr. Syed Gohar Taqi Kazimi


