Reactive intermediates
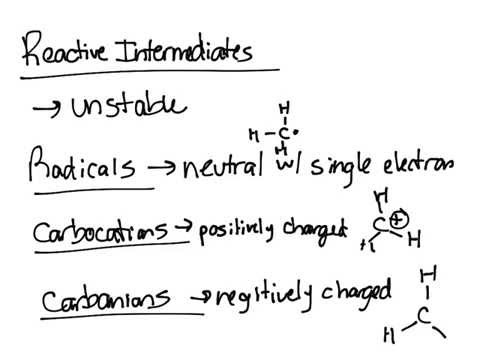
REACTIVE INTERMEDIATES
Many organic reactions involve formation of transient intermediates that have a vital role in the overall processes. These intermediates have lifetimes appreciably longer than a molecular vibration (upward from 10-12 sec.) and most are present (at least in solution) only as intermediates which are converted to more stable molecules. However some are more stable than others. These intermediates may be formed by attack of various reagents on substrate, by dissociation of organic compounds, or by promotion of molecules to excited states by absorption of light or interaction with high energy radiations, such as alpha, beta or gamma rays. The four types of species are carbonium ions, free radicals, carbanions and carbenes. Of the four, only carbanions have a complete octet around the carbon. There are many other organic ions and radicals with charges and unpaired electrons on atoms other than carbon like nitrenes.


