Aromatic Nucleophilic Reactions (diazonium salts)
Arenediazonium Salts
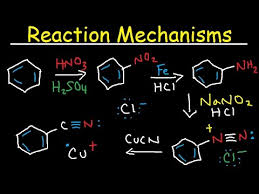
The diazonium salts or diazonium compounds are the class of organic compounds with general formula R−NN+X−. Here R is an alkyl or aryl group, X is an organic or inorganic anion (for example, Cl− Br−, BF4−, etc.).It is formed by the reaction of an aromatic amine with nitrous acid at 0–5°C. Such salts with alkyl amines are unstable and immediately decompose with the evolution of nitrogen. Aromatic diazonium salts are stable at 0° but eliminate N2 at room temperature.
Diazonium compounds are standard reagents used in synthesis of a vast variety organic compounds, especially aryl derivatives. These salts are light sensitive and break down under near UV or violet light. This property has led to their use in document reproduction. In this process, paper or film is coated with a diazonium salt. They also find application in the dye and pigment industries and are used to produce dyed fabrics
- Kinetic control vs thermodynamic control
- Selectivity (stereo, regio, chemo)
- Active methylene compounds
- Aldol Condensation
- Claisen condensation
- Cross Claisen Condensation
- Reformatsky Reaction
- Stobbes Condensation
- Darzen condensation
- Perkin condensation
- Wittig Reaction
- Nucleophilic Substitution Reactions
- SN Reactions I
- SN Reactions II
- SN Reactions III
- Nucleophilic Substitution Prime Reactions
- Neighbouring group participation
- Aromatic Electrophilic Substitution Reactions
- NAS Elimination Addition
- NAS Addition Elimination
- Aromatic Nucleophilic Reactions (diazonium salts)
- Elimination Reactions
- Reactive intermediates
- Chapters 23
- Department Chemistry
- Teacher
Dr. Syed Gohar Taqi Kazimi


