Claisen condensation
Claisen Reaction
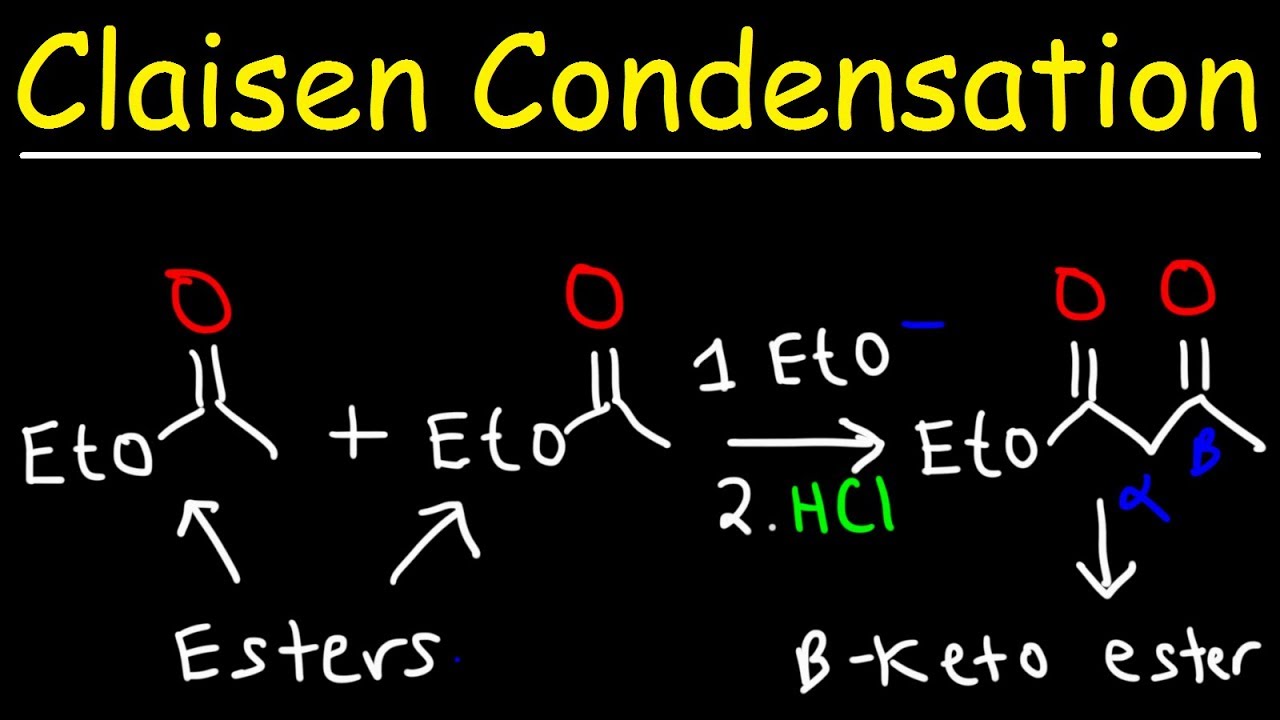
It is a reaction between two molecules of an ester or or one ester and another carbonyl compound in the presence of a strong base like sodium ethoxide, resulting in a β-keto ester or a β-diketone. The reaction is named after its discoverer, the German chemist Rainer Ludwig Claisen.
In case of Claisen Condensation between esters containing α-hydrogens, the driving force is the formation of the stabilized anion of the β-keto ester. If two different esters are used, we get a mixture of all four products, and the preparation does not have high synthetic utility.
However, if one of the ester partners has enolizable α-hydrogens and the other does not (e.g., aromatic esters or carbonates), the mixed reaction (or crossed Claisen) can be synthetically useful. If ketones or nitriles are used as the donor in this condensation reaction, a β-diketone or a β-ketonitrile is obtained, respectively. The use of stronger bases, e.g. sodium amide or sodium hydride instead of sodium ethoxide, often increases the yield.
- Kinetic control vs thermodynamic control
- Selectivity (stereo, regio, chemo)
- Active methylene compounds
- Aldol Condensation
- Claisen condensation
- Cross Claisen Condensation
- Reformatsky Reaction
- Stobbes Condensation
- Darzen condensation
- Perkin condensation
- Wittig Reaction
- Nucleophilic Substitution Reactions
- SN Reactions I
- SN Reactions II
- SN Reactions III
- Nucleophilic Substitution Prime Reactions
- Neighbouring group participation
- Aromatic Electrophilic Substitution Reactions
- NAS Elimination Addition
- NAS Addition Elimination
- Aromatic Nucleophilic Reactions (diazonium salts)
- Elimination Reactions
- Reactive intermediates
- Chapters 23
- Department Chemistry
- Teacher
Dr. Syed Gohar Taqi Kazimi


