week7: Uv/Vis spectroscopy & atomic absorption( principle, instrumentation, sample handling & applications)
Atomic absorption methods measure the amount of energy (in the form of photons of light, and thus a change in the wavelength) absorbed by the sample. Specifically, a detector measures the wavelengths of light transmitted by the sample (the "after" wavelengths), and compares them to the wavelengths, which originally passed through the sample (the "before" wavelengths). A signal processor then integrates the changes in wavelength, which appear in the readout as peaks of energy absorption at discrete wavelengths.
Principle: Any atom has its own distinct pattern of wavelengths at which it will absorb energy, due to the unique configuration of electrons in its outer shell. This allows for the qualitative analysis of a pure sample.In order to tell how much of a known element is present in a sample, one must first establish a basis for comparison using known quantities. It can be done producing a calibration curve. For this process, a known wavelength is selected, and the detector will measure only the energy emitted at that wavelength. However, as the concentration of the target atom in the sample increases, absorption will also increase proportionally. Thus, one runs a series of known concentrations of some compound, and records the corresponding degree of absorbance, which is an inverse percentage of light transmitted. A straight line can then be drawn between all of the known points. From this line, one can then extrapolate the concentration of the substance under investigation from its absorbance. The use of special light sources and specific wavelength selection allows the quantitative determination of individual components of a multielement mixture.
History: The phenomenon of atomic absorption (AA) was first observed in 1802 with the discovery of the Fraunhofer lines in the sun's spectrum. It was not until 1953 that Australian physicist Sir Alan demonstrated that atomic absorption could be used as a quantitative analtical tool. Atomic absorption analysis involves measuring the absorption of light by vaporized ground state atoms and relating the absorption to concentration. The incident light beam is attenuated by atomic vapor absorption according to Beer's law
Procedure: The process of atomic absorption spectroscopy (AAS) involves two steps:
1. Atomization of the sample
2. The absorption of radiation from a light source by the free atoms
The sample, either a liquid or a solid, is atomized in either a flame or a graphite furnace. Upon the absorption of ultraviolet or visible light, the free atoms undergo electronic transitions from the ground state to excited electronic states.
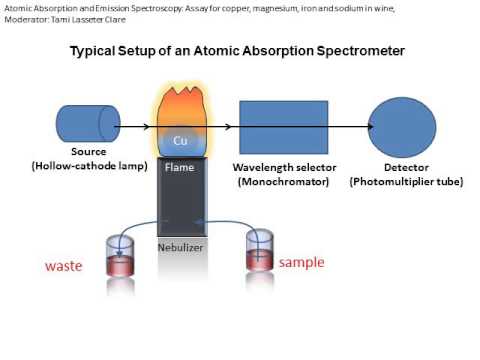
To obtain the best results in AA, the instrumental and chemical parameters of the system must be geared toward the production of neutral ground state atoms of the element of interest. A common method is to introduce a liquid sample into a flame. Upon introduction, the sample solution is dispersed into a fine spray, the spray is then desolvated into salt particles in the flame and the particles are subsequently vaporized into neutral atoms, ionic species and molecular species. All of these conversion processes occur in geometrically definable regions in the flame. It is therefore important to set the instrument parameters such that the light from the source (typically a hollow cathode lamp) is directed through the region of the flame that contains the maximum number of neutral atoms. The light produced by the hollow-cathode lamp is emitted from excited atoms of the same element which is to be determined. Therefore the radiant energy corresponds directly to the wavelength which is absorbable by the atomized sample. This method provides both sensitivity and selectivity since other elements in the sample will not generally absorb the chosen wavelength and thus, will not interfere with the measurement. To reduce background interference, the wavelength of interest is isolated by a monochromator placed between the sample and the detector.
Instrumentation

Figure 2. Perkin-Elmer Spectrophotometer Model 460
there are two methods of adding thermal energy to a sample. A graphite furnace AAS uses a graphite tube with a strong electric current to heat the sample. In flame AAS (see photo above), we aspirate a sample into a flame .The flame is lined up in a beam of light of the appropriate wavelength. The flame (thermal energy) causes the atom to undergo a transition from the ground state to the first excited state. When the atoms make their transition, they absorb some of the light from the beam. The more concentrated the solution, the more light energy is absorbed!
the light beam is generated by lamp that is specific for a target metal. The lamp must be perfectly aligned so the beam crosses the hottest part of the flame. The light passed through the flame is received by the monochromator which is set to accept and transmit radiation at the specified wavelength and travels into the detector. The detector measures the intensity of the beam of light. When some of the light is absorbed by metal, the beam's intensity is reduced. The detector records that reduction as absorption. That absorption is shown on output device by the data system.
Analysis: We can find the concentrations of metals in a sample running a series of calibration standards through the instrument. The instrument will record the absorption generated by a given concentration. By plotting the absorption versus the concentrations of the standards, a calibration curve can be plotted. We can then look at the absorption for a sample solution and use the calibration curves to determine the concentration.


