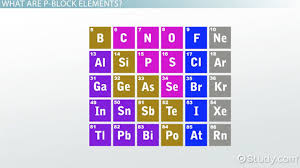
WEEK 10 :P block elements & solar cell
Solar cell, also called photovoltaic cell, any device that directly converts the energy of light into electrical energy through the photovoltaic effect.
The overwhelming majority of solar cells are fabricated from silicon—with increasing efficiency and lowering cost as the materials range from amorphous (noncrystalline) to polycrystalline to crystalline (single crystal) silicon forms.
Unlike batteries or fuel cells, solar cells do not utilize chemical reactions or require fuel to produce electric power, and, unlike electric generators, they do not have any moving parts.
Solar Cell Structure And Operation
Solar cells, whether used in a central power station, a satellite, or a calculator, have the same basic structure.
• Light enters the device through an optical coating, or antireflection layer, that minimizes the loss of light by reflection;
• it effectively traps the light falling on the solar cell by promoting its transmission to the energy-conversion layers below.
• The antireflection layer is typically an oxide of
silicon, tantalum, or titanium
that is formed on the cell surface by spin-coating or a vacuum deposition technique.
PRINCIPLE
When light falls on a solar cell, electrons in the absorber layer are excited from a lower-energy “ground state,” in which they are bound to specific atoms in the solid, to a higher “excited state,” in which they can move through the solid. In the absence of the junction-forming layers, these “free” electrons are in random motion, and so there can be no oriented direct current. The addition of junction-forming layers, however, induces a built-in electric field that produces the photovoltaic effect. In effect, the electric field gives a collective motion to the electrons that flow past the electrical contact layers into an external circuit where they can do useful work.
The materials used for the two junction-forming layers must be dissimilar to the absorber in order to produce the built-in electric field and to carry the electric current. Hence, these may be different semiconductors (or the same semiconductor with different types of conduction), or they may be a metal and a semiconductor. The materials used to construct the various layers of solar cells are essentially the same as those used to produce the diodes and transistors of solid-state electronics and microelectronics (see also electronics: Optoelectronics). Solar cells and microelectronic devices share the same basic technology. In solar cell fabrication, however, one seeks to construct a large-area device because the power produced is proportional to the illuminated area. In microelectronics the goal is, of course, to construct electronic components of ever smaller dimensions in order to increase their density and operating speed within semiconductor chips, or integrated circuits.
The photovoltaic process bears certain similarities to photosynthesis, the process by which the energy in light is converted into chemical energy in plants. Since solar cells obviously cannot produce electric power in the dark, part of the energy they develop under light is stored, in many applications, for use when light is not available. One common means of storing this electrical energy is by charging electrochemical storage batteries. This sequence of converting the energy in light into the energy of excited electrons and then into stored chemical energy is strikingly similar to the process of photosynthesis.


