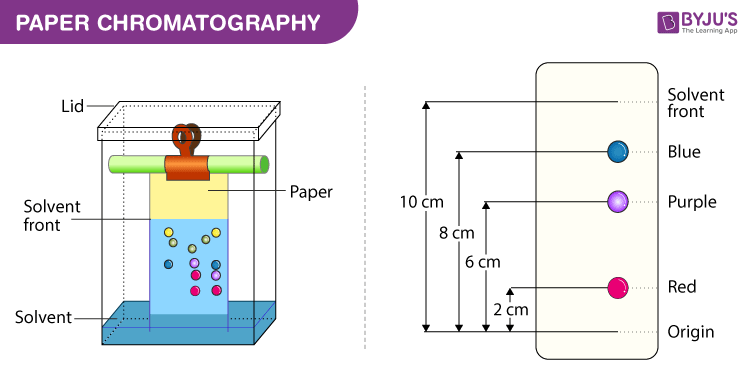
week4:paper chromatography principle, applications
Principle of paper chromatography: The principle involved is partition chromatography wherein the substances are distributed or partitioned between liquid phases. One phase is the water, which is held in the pores of the filter paper used; and other is the mobile phase which moves over the paper. The compounds in the mixture get separated due to differences in their affinity towards water (in stationary phase) and mobile phase solvents during the movement of mobile phase under the capillary action of pores in the paper.
The principle can also be adsorption chromatography between solid and liquid phases, wherein the stationary phase is the solid surface of the paper and the liquid phase is of the mobile phase. But most of the applications of paper chromatography work on the principle of partition chromatography, i.e., partitioned between to liquid phases.
Uses and Applications of Paper Chromatography
Paper chromatography is specially used for the separation of a mixture having polar and non-polar compounds.
For separation of amino acids.
It is used to determine organic compounds, biochemicals in urine, etc.
In the pharma sector, it is used for the determination of hormones, drugs, etc.
Sometimes it is used for evaluation of inorganic compounds like salts and complexes.
Ascending Paper chromatography

Ascending type
Radial paper chromatography

Types or Modes of Paper Chromatography
Based on the way the development of chromatogram on paper is done in procedures, we have, broadly, five types of chromatography.
1. Ascending chromatography: As the name indicates, the chromatogram ascends. Here, the development of paper occurs due to the solvent movement or upward travel on the paper.
The solvent reservoir is at the bottom of the beaker. The paper tip with sample spots just dips into the solvent at the bottom so that spots remain well above the solvent.
2. Descending chromatography: Here, the development of paper occurs due to solvent travel downwards on the paper.
The solvent reservoir is at the top. The movement of the solvent is assisted by gravity besides the capillary action.
3. Ascending- descending mode: Here solvent first travels upwards and then downwards on the paper.
4. Radial mode: Here, the solvent moves from the center (mid-point) towards the periphery of circular chromatography paper. The entire system is kept in a covered Petri dish for the development of the chromatogram.
The wick at the center of paper dips into the mobile phase in a petri dish, by which the solvent drains on to the paper and moves the sample radially to form the sample spots of different compounds as concentric rings.
Paper Chromatography Experiment Method
The experimental method involves:
a) Selection of suitable type of development: This depends on the complexity of the mixture, solvent, paper, etc. But in general ascending type or radial type chromatography are used as they are easy to perform, handle, less time-consuming and also give chromatogram faster.
b) Selection of suitable filter paper: Filter paper is selected based on pore size, the quality of the sample to be separated, and also the mode of development.
c) Preparation of sample: Preparation of the sample involves the dissolution of the sample in a suitable solvent used in making mobile phase. The solvent used should be inert with the sample under analysis.
d) Spotting of sample on the paper: Samples are to be spotted at the proper position on the paper, preferably using a capillary tube.
d) Development of chromatogram: Sample spotted paper is subjected to development by immersing it in the mobile phase. The mobile phase moves over the sample on the paper under the capillary action of paper.
e) Drying of the paper and detection of the compounds: Once the development of chromatogram is over; the paper is held carefully at the borders to avoid touching the sample spots and dried using an air drier. Sometimes the detecting solution is sprayed in the developed paper and dried to identify the sample chromatogram spots.


