week 1 Periodic Table, Group trends and periodic properties, Atomic & ionic radii
The periodic table we use today is based on the one devised and published by Dmitri Mendeleev in 1869.Mendeleev found he could arrange the 65 elements then known in a grid or table so that each element had:
1. A higher atomic weight than the one on its left. For example, magnesium (atomic weight 24.3) is placed to the right of sodium (atomic weight 23.0):
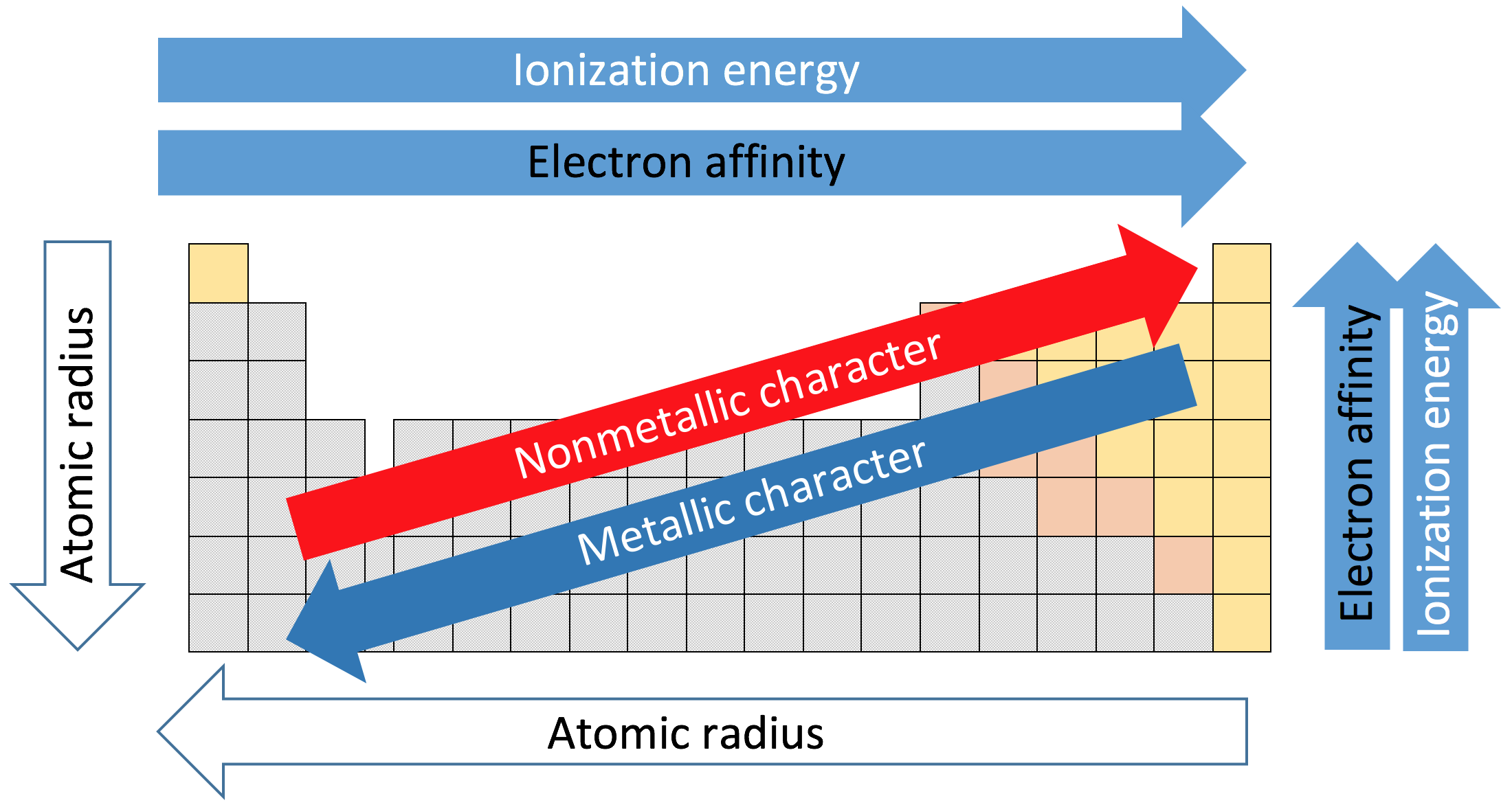
Today the chemical elements are still arranged in order of increasing atomic number (Z) as we look from left to right across the periodic table. We call the horizontal rows periods.
We call the vertical rows groups. We also now know that an element's chemistry is determined by the way its electrons are arranged - its electron configuration.


