Week2 :ionization potentials, electron affinities and electronegativities; Redox potential, electrochemical series and its applications
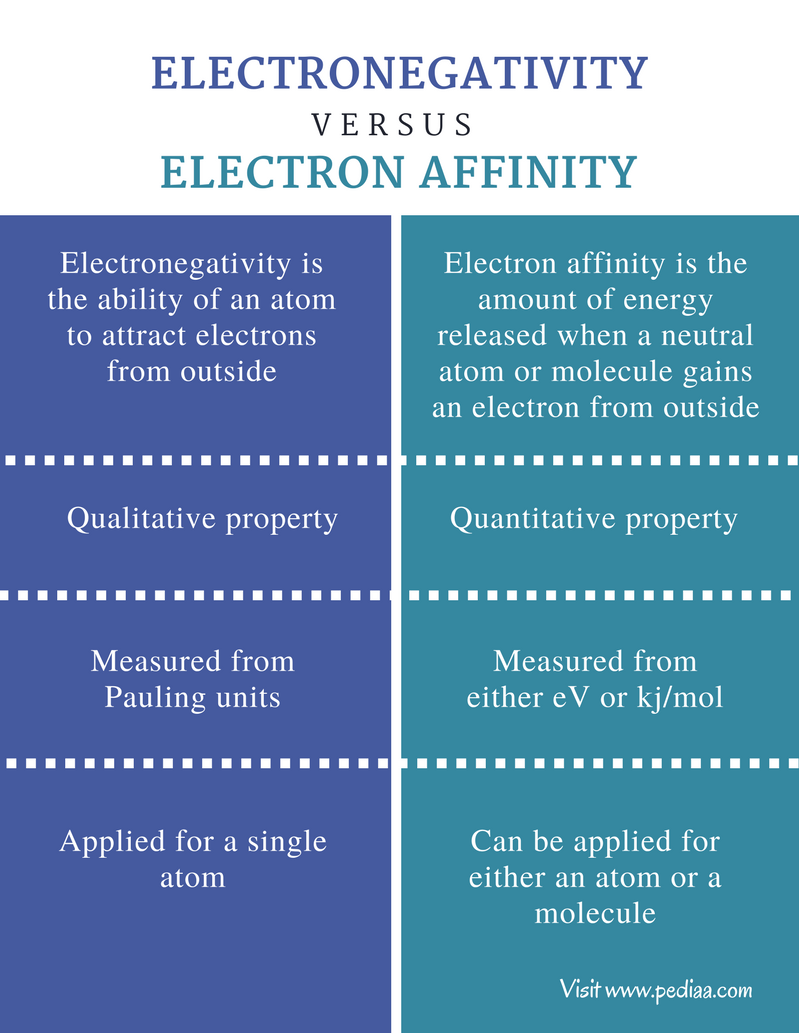
Electronegativity is the tendency of an atom to attract electrons toward itself when forming bonds.Electronegativity decreases from top to bottom down the periodic table. This happens because the atoms of elements lower in a group have a larger atomic radius and thus an increased distance between the valence electrons and the nucleus. As a result, atoms with a larger atomic radius exert a weaker force on nearby atoms. They are therefore less likely to form bonds with other elements compared to atoms with a smaller atomic radius
.Electron affinity is the change in energy that occurs when an atom of a neutral gas gains an electron. It is a quantitative attribute, and thus can be measured numerically. Electron affinity indicates the ability of an atom to accept an electron. It increases from left to right across the periodic table and decreases from top to bottom down the periodic table. These trends are due to atomic radius: a smaller atomic radius is associated with a higher electron affinity, because less energy is required to add electrons to a valence shell closer to the nucleus compared to one that is farther away. However, electron affinities are difficult to measure accurately, so the trends are a generalization.


