week 5: column chromatography (principle. procedure, application)
Column chromatography is a chromatography technique used to separate mixture of chemical substances into its individual compounds. Column chromatography is a widely used method for the purification or separation of chemical compound mixture in lab.
Principles of column chromatography
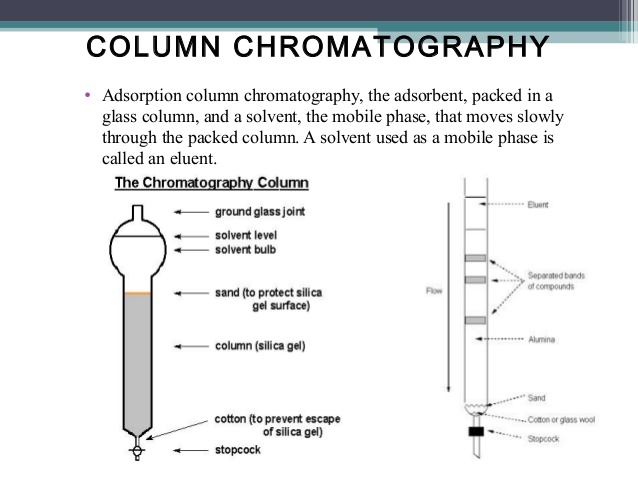
Column Chromatography consists of two phases: one mobile phase and one contiguous stationery phase. The stationery phase is solid and the mobile phase is liquid. The compound mixture moves along with the mobile phase through stationery phase and separates depending on the different degree of adhesion (to the silica) of each component in the sample or the compound mixture.
Explanation
The stationery phase
A glass tube with a circle large inlet and a small outlet with a plug or tap, named as column is used for this column chromatography. The column is placed vertically with a stand where the outlet is downward.
![Column chromatography 2 By http://www.tutorvista.com/content/chemistry/chemistry-iii/organic-compounds/mass.php [CC0], via Wikimedia Commons](https://upload.wikimedia.org/wikipedia/commons/0/09/Column_chromatography.jpg)
A piece of cotton wool is entered into the outlet and placed over the plug if there are no glass wool present to stop escaping the stationery phase from the column. There are two procedure to prepare the column by packing with silica or alumina:
Dry method: In dry method at first the column is filled with dry powdered silica. Then the mobile phase, a suitable solvent is flushed through it until all the silica are wet and settled. From this point till the end always the column need to keep wet with solvent.
Wet method: In wet method firstly a slurry of silica and solvent is prepared and then poured onto the column using a funnel. More solvent must be used until the silica is settled into it.
Process
Column chromatography works in few steps:
Step 1: The mobile phase or eluent is either solvent or solvent mixture. The upper level of mobile phase should be same as the stationery phase. That means the stationery phase should be wet with the solvent. On this stage the compound mixture what need to be separated, are added from the top of the column in such a way that the top level of it is not disturbed. By turning on the tap below it is allowed to adsorbed on the surface of the silica.
![Column chromatography 3 By EjupPod (Own work) [CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0)], via Wikimedia Commons](https://upload.wikimedia.org/wikipedia/commons/4/4c/Kromatografia.png)
Step 2: Then the solvent or a suitable solvent mixture is added at first touching the side of the glass column slowly and carefully so that the top level of the stationery phase is not disturbed. The solvent is repeatedly added as many times as needed throughout the process.
Step 3: When the tap, is on the compounds in the compound mixture move along with the eluent depending on the polarity of the sample molecule. The non polar components travel faster than the polar component.
Suppose if any compound mixture contains three compounds blue, red and green. According to polarity the order of these compounds are blue>red>green. That means blue is the most polar compound and thus will have less tendency to move along with the mobile phase.
Step 4: The green colored compound will travel first as it is less polar that other two. When it is near end of the column a clean test tube is taken to collect the green sample. After this the red and at last the most polar blue compound is collected, all in separate test tubes.
Thus a compound mixture is separated or purified by using column chromatography.
Summary
- Column layer chromatography is an chromatography technique used to separate mixture of chemical substances into its individual compounds.
- Chromatography consists of two phases: one mobile phase and one contiguous stationery phase.
- Column is prepared my mixing the silica with suitable solvent and poured in into a glass column.
- A suitable solvent (mobile phase) is moved along with compound mixture through the column according to the polarity.


