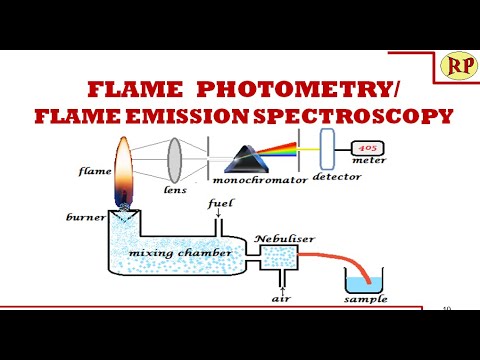
week 8: Infrared & Fame emission spectroscopic techniques 9principle, instrumentation, sample handling , applications)
Flame atomic emission spectroscopy (FAES) is a classical method which has been largely displaced by plasma spectroscopies. Plasmas produce higher atomization ratios, but the theory is similar in both flame and the plasmas. FAES is the classical method used as plasmas have taken over as the preferred method due to the higher atomization ratios that occur. Using the flame could be advantageous in a group 1 & 2 elemental analysis since less ionization will occur at lower temperatures (compared to a plasma). It is typically not used often, unless sensitivity and cost are possible issues.
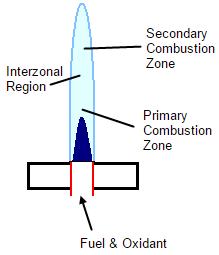
Below is a very simple schematic for a laminar flow burner. The Primary Combustion Zone is where the initial decomposition occurs and molecular fragments are observed. The Interzonal Region is the hottest part of the flame and atomic fragment are observed. The Secondary Combustion Zone is cooler overall and a conversion is seen from atoms back to stable molecules and oxides.
Fuel (usually acetylene) and air are added mixed with a nebulizer mist. This mixture is then introduced into the flame.
The advantages to the Laminar flow burner are that it is cheap, simple, relatively stable and can operate at lower temperatures. An issue that complicates flame emission just as it complicates plasma emission is self reversal.
procedure: The process consists of four interdependent steps:
(1) vaporization of the sample,
(2) electronic excitation of its atoms or ions,
(3) dispersion of the emitted or absorbed radiation into its component frequencies,
(4) measurement of the intensity of the radiation, usually at wavelengths at which the intensity is greatest.
APPLICATIONS
Most applications of FES have been the determination of trace metals, especially in liquid samples. It shoul dbe remembered that FES offers a simple, inexpensive, and sensitive method for detecting common metals, including the alkali and alkaline earths, as well as several transition metals such as Fe, Mn, Cu, and Zn. FES has been extended to include a number of nonmetals: H, B, C, N, P, As, O, S, Se, Te, halogens, and noble gases. FES detectors for P and S are commercially available for use in gas chromatography. FES has found wide application in agricultural and environmental analysis, industrial analyses of ferrous metals and alloys as well as glasses and ceramic materials, and clinical analyses of body fluids. FES can be easily automated to handle a large number of samples. Array detectors interfaced to a microcomputer system permit simultaneous analyses of several elements in a single sample.


