optical isomerism
Optical isomers
Those isomers which have same molecular formula and same functional group (i.e., the connectivity between atoms is the same), but different spatial arrangements of the atoms, and have non-superimposable mirror images. Each non-superimposable mirror image structure is called an enantiomer.
Optical Activity
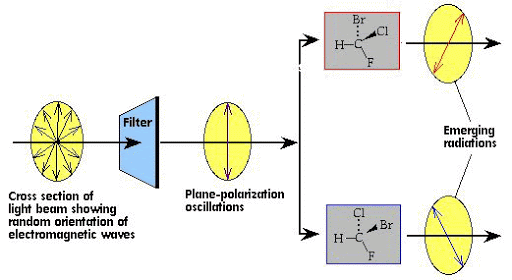
Optical isomers can occur when there is an asymmetric carbon atom. An asymmetric carbon atom is one which is bonded to four different groups. It forms a chiral centre of the molecule. The four groups can be something hideously complex, or something comfortably simple like a hydrogen atom or a chlorine atom. Optical activity is the ability of a chiral molecule to rotate the plane of plane-polairsed light, measured using a polarimeter. A simple polarimeter consists of a light source, polarising lens, sample tube and analysing lens. A solution of chiral compounds - light emerges with its plane of polarization changed - the solution is optically active and rotates the plane of polarized light clockwise or counterclockwise.
Dextrorotatory (+) compounds rotate plane polarized light clockwise Latin - dextro - “to the right”(d).
Levorotatory (-) compounds rotate plane polarized light counter clockwise Latin - levo - “to the left”(l).
- Resonance
- Resonance Effect
- Dipole Moment
- Iductive effect
- Hydrogen Bonding
- Nomenclature of Organic Compounds
- Alkanes and Cycloalkanes
- Alkenes and Alkynes
- Wittig Reaction
- Isomerism
- Isomerism I
- optical isomerism
- Aromatic Electrophilic Substitution Reactions
- Nucleophilic Substitution Reactions I
- Nucleophilic Substitution Reactions II
- Nucleophilic Substitution Reactions III
- Alcohols 1
- Alcohols 2
- Carboxylic Acid 1
- Carboxylic Acid 2
- Aldehyde and ketone
- Base catalyzed reactions of carbonyl compounds I
- Base catalyzed reactions of carbonyl compounds II
- Aldol Condensation
- Reactions of alkyl amines, Arene diazonium salts
- Unsaturated Hydrocarbons
- Chapters 26
- Department Biotechnology
- Teacher
Dr. Syed Gohar Taqi Kazimi


