Hydrogen Bonding
Hydrogen bonding
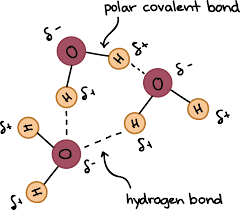
• Hydrogen bond or hydrogen bonding is a type of weak force that arises due to interaction between a partial positive hydrogen atom of one molecule and highly electronegative atom of other molecule. The hydrogen bonding is strong in comparison to normal dipole-dipole and dispersion forces. However, they are weak compared to true covalent or ionic bonds.
• Hydrogen Bonding in Hydrogen fluoride
Fluorine having the highest value of electronegativity forms the strongest hydrogen bond.
•Hydrogen Bonding in Water
A water molecule contains a highly electronegative oxygen atom linked to the hydrogen atom. Oxygen atom attracts the shared pair of electrons more and this end of the molecule becomes negative whereas the hydrogen atoms become positive.


