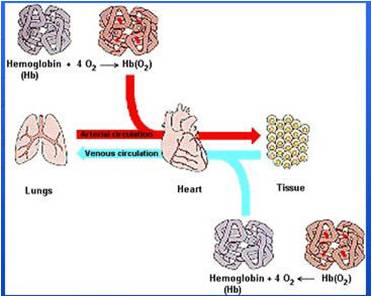
WEEK 16-20:Transport Of O2 & CO2 In Blood
Once oxygen has diffused from the alveoli into
the pulmonary blood, it is transported to the
peripheral tissue capillaries almost entirely in combination
with hemoglobin. The presence of hemoglobin
in the red blood cells allows the blood to
transport 30 to 100 times as much oxygen as could
be transported in the form of dissolved oxygen in
the water of the blood.
In the body’s tissue cells, oxygen reacts with various foodstuffs to form large
quantities of carbon dioxide. This carbon dioxide enters the tissue capillaries
and is transported back to the lungs. Carbon dioxide, like oxygen, also combines
with chemical substances in the blood that increase carbon dioxide transport
15- to 20-fold.
The purpose of this chapter is to present both qualitatively and quantitatively
the physical and chemical principles of oxygen and carbon dioxide transport in
the blood and tissue fluids.Transport of Oxygen from the Lungs to the
Body Tissues
In Chapter 39, we pointed out that gases can move from one point to another
by diffusion and that the cause of this movement is always a partial pressure
difference from the first point to the next.Thus, oxygen diffuses from the alveoli
into the pulmonary capillary blood because the oxygen partial pressure (Po2)
in the alveoli is greater than the Po2 in the pulmonary capillary blood. In the
other tissues of the body, a higher Po2 in the capillary blood than in the tissues
causes oxygen to diffuse into the surrounding cells.
Conversely, when oxygen is metabolized in the cells to form carbon dioxide,
the intracellular carbon dioxide pressure (Pco2) rises to a high value, which
causes carbon dioxide to diffuse into the tissue capillaries. After blood flows to
the lungs, the carbon dioxide diffuses out of the blood into the alveoli, because
the Pco2 in the pulmonary capillary blood is greater than that in the alveoli.
Thus, the transport of oxygen and carbon dioxide by the blood depends on both
diffusion and the flow of blood.When oxygen is used by the cells, virtually all of it
becomes carbon dioxide, and this increases the intracellular
Pco2; because of this high tissue cell Pco2,
carbon dioxide diffuses from the cells into the tissue
capillaries and is then carried by the blood to the lungs.
In the lungs, it diffuses from the pulmonary capillaries
into the alveoli and is expired.
Thus, at each point in the gas transport chain, carbon
dioxide diffuses in the direction exactly opposite to the
diffusion of oxygen.Yet there is one major difference
between diffusion of carbon dioxide and of oxygen:
carbon dioxide can diffuse about 20 times as rapidly as
oxygen.Therefore, the pressure differences required to
cause carbon dioxide diffusion are, in each instance, far
less than the pressure differences required to cause
oxygen diffusion. The CO2 pressures are approximately
the following:
1. Intracellular Pco2, 46 mm Hg; interstitial Pco2,
45 mm Hg. Thus, there is only a 1 mm Hg pressure
differential, as shown in Figure 40–5.
2. Pco2 of the arterial blood entering the tissues,
40 mm Hg; Pco2 of the venous blood leaving the
tissues, 45 mm Hg. Thus, as shown in Figure 40–5,
the tissue capillary blood comes almost exactly
to equilibrium with the interstitial Pco2 of
45 mm Hg.
3. Pco2 of the blood entering the pulmonary
capillaries at the arterial end, 45 mm Hg; Pco2 of
the alveolar air, 40 mm Hg. Thus, only a 5 mm Hg
pressure difference causes all the required carbon
dioxide diffusion out of the pulmonary capillariesRespiratory Exchange Ratio
The discerning student will have noted that normal
transport of oxygen from the lungs to the tissues by each
100 milliliters of blood is about 5 milliliters, whereas
normal transport of carbon dioxide from the tissues
to the lungs is about 4 milliliters. Thus, under normal
resting conditions, only about 82 per cent as much
carbon dioxide is expired from the lungs as oxygen is
taken up by the lungs. The ratio of carbon dioxide
output to oxygen uptake is called the respiratory
exchange ratio (R). That is,
The value for R changes under different metabolic
conditions.When a person is using exclusively carbohydrates
for body metabolism, R rises to 1.00. Conversely,
when a person is using exclusively fats for metabolic
energy, the R level falls to as low as 0.7. The reason
for this difference is that when oxygen is metabolized
with carbohydrates, one molecule of carbon dioxide is
formed for each molecule of oxygen consumed; when
oxygen reacts with fats, a large share of the oxygen
combines with hydrogen atoms from the fats to form
water instead of carbon dioxide. In other words, when
fats are metabolized, the respiratory quotient of the
chemical reactions in the tissues is about 0.70 instead of
1.00. (The tissue respiratory quotient is discussed in
Chapter 71.) For a person on a normal diet consuming
average amounts of carbohydrates, fats, and proteins.


