WEEK 11-15:Diffusion of Gases through Respiratory Membrane
All the gases of concern in respiratory physiology are simple molecules that are free
to move among one another, which is the process called “diffusion.”This is also true
of gases dissolved in the fluids and tissues of the body.
For diffusion to occur, there must be a source of energy. This is provided by the
kinetic motion of the molecules themselves. Except at absolute zero temperature,
all molecules of all matter are continually undergoing motion. For free molecules
that are not physically attached to others, this means linear movement at high velocity
until they strike other molecules. Then they bounce away in new directions and
continue until striking other molecules again. In this way, the molecules move
rapidly and randomly among one anotherNet Diffusion of a Gas in One Direction—Effect of a Concentration Gradient. If a gas chamber
or a solution has a high concentration of a particular gas at one end of the chamber
and a low concentration at the other end,net diffusion of
the gas will occur from the high-concentration area toward the low-concentration
area.The reason is obvious:There are far more molecules at end A of the chamber
to diffuse toward end B than there are molecules to diffuse in the opposite direction.
Therefore, the rates of diffusion in each of the two directions are proportionately
different, as demonstrated by the lengths of the arrows in the figure.Gas Pressures in a Mixture of Gases—“Partial Pressures”
of Individual Gases
Pressure is caused by multiple impacts of moving molecules against a surface.Therefore,
the pressure of a gas acting on the surfaces of the respiratory passages and
alveoli is proportional to the summated force of impact of all the molecules of that
gas striking the surface at any given instant. This means that the pressure is directlyproportional to the concentration of the gas moleculesthe respiratory unit
(also called “respiratory lobule”), which is composed
of a respiratory bronchiole, alveolar ducts, atria, and
alveoli. There are about 300 million alveoli in the two
lungs, and each alveolus has an average diameter of
about 0.2 millimeter. The alveolar walls are extremely
thin, and between the alveoli is an almost solid
network of interconnecting capillaries,Indeed, because of the extensiveness of
the capillary plexus, the flow of blood in the alveolar
wall has been described as a “sheet” of flowing blood.
Thus, it is obvious that the alveolar gases are in very
close proximity to the blood of the pulmonary capillaries.
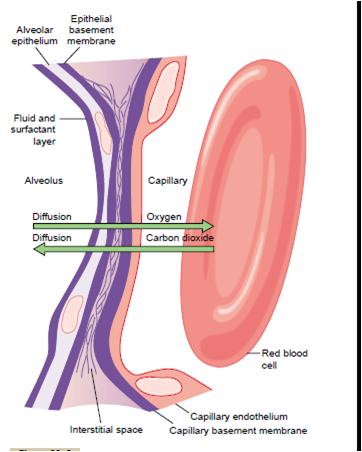
Further, gas exchange between the alveolar airNote the following different
layers of the respiratory membrane:
1. A layer of fluid lining the alveolus and containing
surfactant that reduces the surface tension of the
alveolar fluid
2. The alveolar epithelium composed of thin
epithelial cells
3. An epithelial basement membrane
4. A thin interstitial space between the alveolar
epithelium and the capillary membrane5. A capillary basement membrane that in many
places fuses with the alveolar epithelial basement
membrane
6. The capillary endothelial membrane
Despite the large number of layers, the overall
thickness of the respiratory membrane in some areas
is as little as 0.2 micrometer, and it averages about 0.6
micrometer, except where there are cell nuclei. From
histological studies, it has been estimated that the total
surface area of the respiratory membrane is about 70
square meters in the normal adult human male.This is
equivalent to the floor area of a 25–by-30–foot room.
The total quantity of blood in the capillaries of the
lungs at any given instant is 60 to 140 milliliters. Now
imagine this small amount of blood spread over the
entire surface of a 25–by-30–foot floor, and it is easy
to understand the rapidity of the respiratory exchange
of oxygen and carbon dioxide.
The average diameter of the pulmonary capillaries
is only about 5 micrometers, which means that red
blood cells must squeeze through them.The red blood
cell membrane usually touches the capillary wall, so
that oxygen and carbon dioxide need not pass through
significant amounts of plasma as they diffuse between
the alveolus and the red cell. This, too, increases the
rapidity of diffusion


