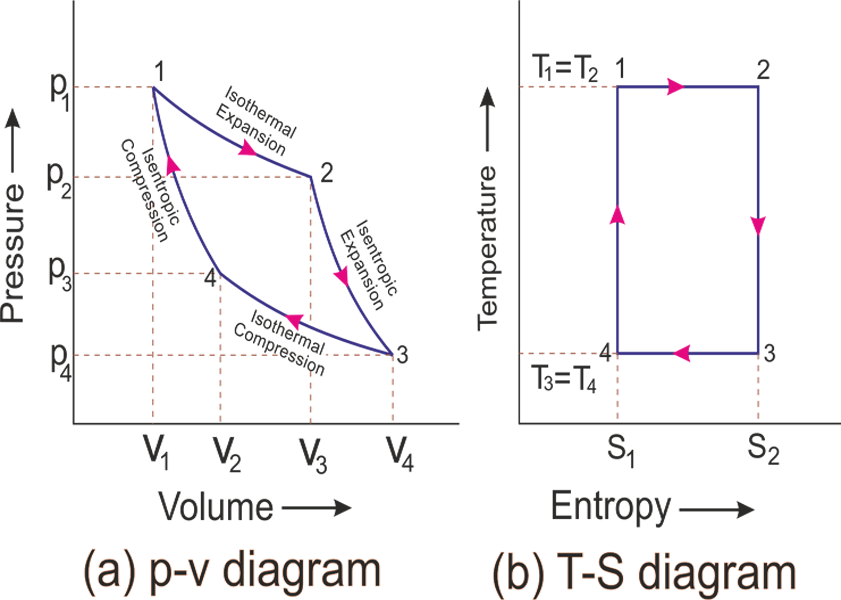
Week 6: Calculation of Work Done, Consequences of the First Law of Thermodynamics
The laws of thermodynamics are deceptively simple to state, but they are far-reaching in their consequences. The first law asserts that if heat is recognized as a form of energy, then the total energy of a system plus its surroundings is conserved; in other words, the total energy of the universe remains constant.The first law of thermodynamics states that to produce a definite amount of work, one must expend an equal amount of energy. This is the assertion that the perpetual machines of the first kind do not exist. Any machine which works without expenditure of energy is called the perpetual machine of the first kind. The first law of thermodynamics states that perpetual machines of the first kind don't exist. Although, apparently first law of thermodynamics is so obvious from what we learn in middle school, it's mathematical formulation a powerful tool for working with problems in thermodynamics and deriving useful results in thermodynamics.


