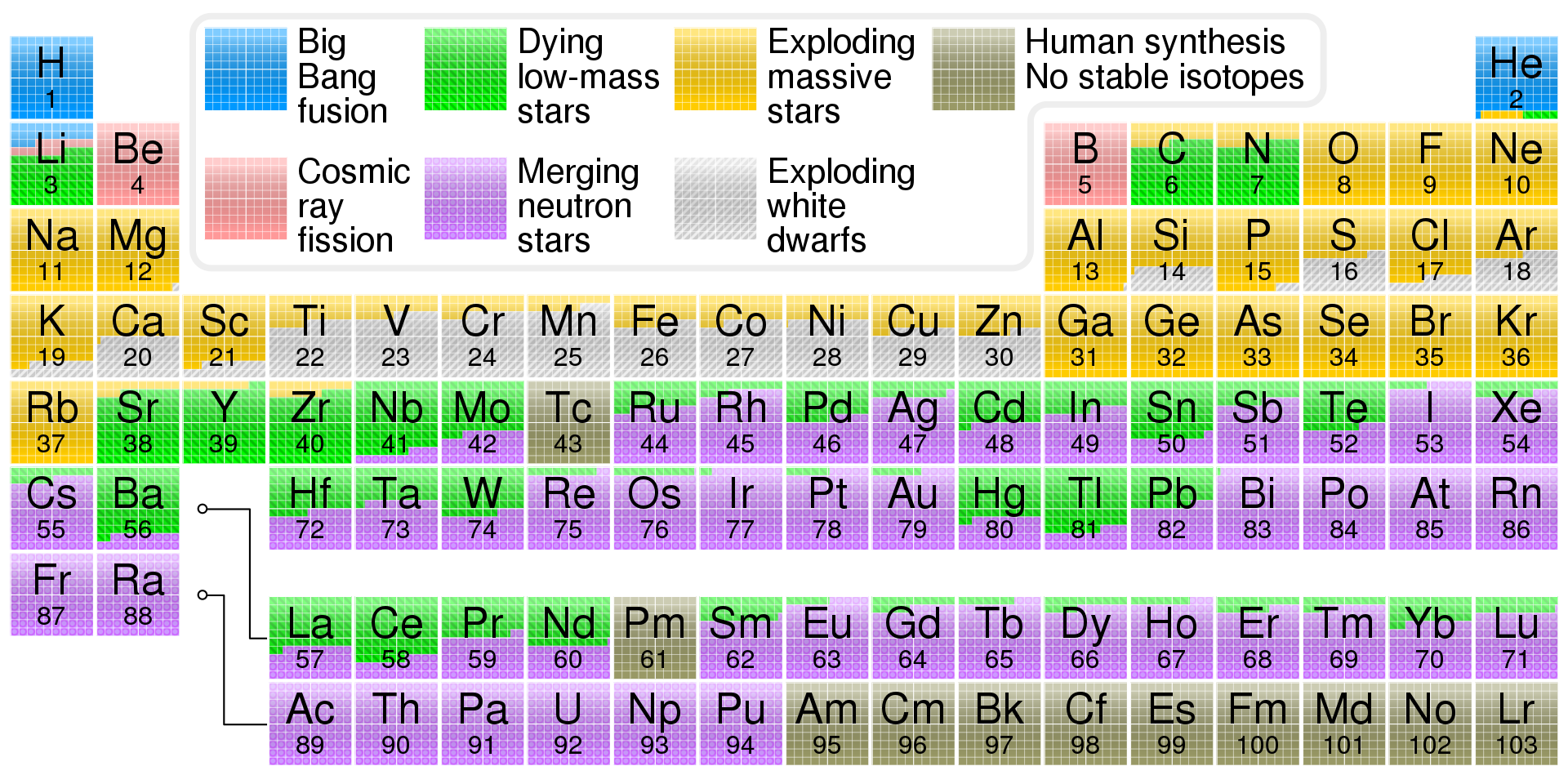
Week 2-3. Origin and Cosmic abundance of elements
Origin of the elements:
Only about 4% of the total mass of the universe is made of atoms or ions, and thus represented by chemical elements. This fraction is about 15% of the total matter, with the remainder of the matter (85%) being dark matter. The nature of dark matter is unknown, but it is not composed of atoms of chemical elements because it contains no protons, neutrons, or electrons.
The universe's 94 naturally occurring chemical elements are thought to have been produced by at least four cosmic processes. Most of the hydrogen, helium and a very small quantity of lithium in the universe was produced primordially in the first few minutes of the Big Bang. Other three recurrently occurring later processes are thought to have produced the remaining elements. Stellar nucleosynthesis, an ongoing process inside stars, produces all elements from carbon through iron in atomic number, but little lithium, beryllium, or boron. Elements heavier in atomic number than iron, as heavy as uranium and plutonium, are produced by explosive nucleosynthesis in supernovas and other cataclysmic cosmic events. Cosmic ray spallation (fragmentation) of carbon, nitrogen, and oxygen is important to the production of lithium, beryllium and boron.
During the early phases of the Big Bang, nucleosynthesis of hydrogen nuclei resulted in the production of hydrogen-1 (protium, 1H) and helium-4 (4He), as well as a smaller amount of deuterium (2H) and very minuscule amounts (on the order of 10−10) of lithium and beryllium. Even smaller amounts of boron may have been produced in the Big Bang, since it has been observed in some very old stars, while carbon has not. It is generally agreed that no heavier elements than boron were produced in the Big Bang. As a result, the primordial abundance of atoms (or ions) consisted of roughly 75% 1H, 25% 4He, and 0.01% deuterium, with only tiny traces of lithium, beryllium, and perhaps boron. Subsequent enrichment of galactic halos occurred due to stellar nucleosynthesis and supernova nucleosynthesis. However, the element abundance in intergalactic space can still closely resemble primordial conditions, unless it has been enriched by some means.
On Earth (and elsewhere), trace amounts of various elements continue to be produced from other elements as products of nuclear transmutation processes. These include some produced by cosmic rays or other nuclear reactions and others produced as decay products of long-lived primordial nuclides. For example, trace amounts of carbon-14 (14C) are continually produced in the atmosphere by cosmic rays impacting nitrogen atoms, and argon-40 (40Ar) is continually produced by the decay of primordially occurring but unstable potassium-40 (40K). Also, three primordially occurring but radioactive actinides, thorium, uranium, and plutonium, decay through a series of recurrently produced but unstable radioactive elements such as radium and radon, which are transiently present in any sample of these metals or their ores or compounds. Three other radioactive elements, technetium, promethium, and neptunium, occur only incidentally in natural materials, produced as individual atoms by nuclear fission of the nuclei of various heavy elements or in other rare nuclear processes.
Periodic table showing the cosmogenic origin of each element in the Big Bang, or in large or small stars. Small stars can produce certain elements up to sulfur, by the alpha process. Supernovae are needed to produce "heavy" elements (those beyond iron and nickel) rapidly by neutron buildup, in the r-process. Certain large stars slowly produce other elements heavier than iron, in the s-process; these may then be blown into space in the off-gassing of planetary nebulae.
Abundance of the chemical elements:
The abundance of elements in the Solar System is in keeping with their origin from nucleosynthesis in the Big Bang and a number of progenitor supernova stars. Very abundant hydrogen and helium are products of the Big Bang, but the next three elements are rare since they had little time to form in the Big Bang and are not made in stars (they are, however, produced in small quantities by the breakup of heavier elements in interstellar dust, as a result of impact by cosmic rays). Beginning with carbon, elements are produced in stars by buildup from alpha particles (helium nuclei), resulting in an alternatingly larger abundance of elements with even atomic numbers (these are also more stable). In general, such elements up to iron are made in large stars in the process of becoming supernovas. Iron-56 is particularly common, since it is the most stable element that can easily be made from alpha particles (being a product of decay of radioactive nickel-56, ultimately made from 14 helium nuclei). Elements heavier than iron are made in energy-absorbing processes in large stars, and their abundance in the universe (and on Earth) generally decreases with their atomic number.
The abundance of the chemical elements on Earth varies from air to crust to ocean, and in various types of life. The abundance of elements in Earth's crust differs from that in the Solar System (as seen in the Sun and heavy planets like Jupiter) mainly in selective loss of the very lightest elements (hydrogen and helium) and also volatile neon, carbon (as hydrocarbons), nitrogen and sulfur, as a result of solar heating in the early formation of the solar system. Oxygen, the most abundant Earth element by mass, is retained on Earth by combination with silicon. Aluminum at 8% by mass is more common in the Earth's crust than in the universe and solar system, but the composition of the far more bulky mantle, which has magnesium and iron in place of aluminum (which occurs there only at 2% of mass) more closely mirrors the elemental composition of the solar system, save for the noted loss of volatile elements to space, and loss of iron which has migrated to the Earth's core.

Abundances of the chemical elements in the Solar System. Hydrogen and helium are most common, from the Big Bang. The next three elements (Li, Be, B) are rare because they are poorly synthesized in the Big Bang and also in stars. The two general trends in the remaining stellar-produced elements are: (1) an alternation of abundance in elements as they have even or odd atomic numbers and (2) a general decrease in abundance as elements become heavier. Iron is especially common because it represents the minimum energy nuclide that can be made by fusion of helium in supernovae.



