Week 9-10. Geo-chemical classification of elements
Goldschmidt Classification:
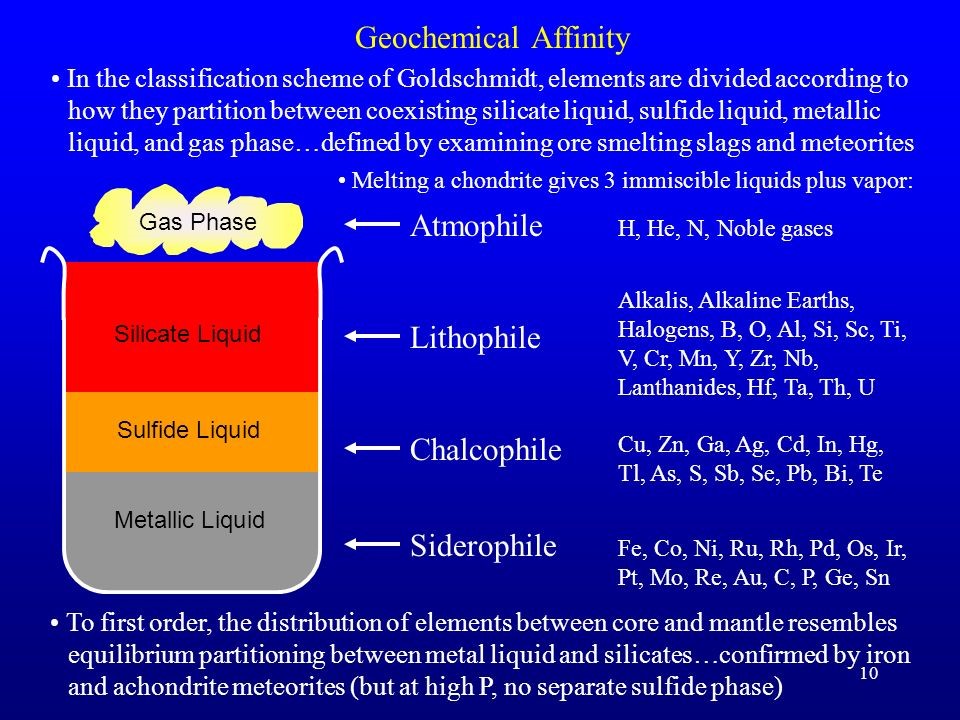
The Goldschmidt classification developed by Victor Goldschmidt (1888–1947), is a geochemical classification which groups the chemical elements within the Earth according to their preferred host phases into lithophile (rock-loving), siderophile (iron-loving), chalcophile (ore-loving or chalcogen-loving), and atmophile (gas-loving) or volatile (the element, or a compound in which it occurs, is liquid or gaseous at ambient surface conditions).
Some elements have affinities to more than one phase. The main affinity is given in the table attached below.
1. Lithophile (rock-loving)
2. Siderophile (iron-loving)
3. Chalcophile (ore-loving or chalcogen-loving)
4. Atmophile (gas-loving) or volatile.
Geochemical Affinity
Affinity of an element for a particular environment.
1. Lithophile Elements
- Lithophile elements are those that remain on or close to the surface because they combine readily with oxygen, forming compounds that do not sink into the core.
- The lithophile elements include: Al, B, Ba, Be, Br, Ca, Cl, Cr, Cs, F, I, Hf, K, Li, Mg, Na, Nb, O, P, Rb, Sc, Si, Sr, Ta, Th, Ti, U, V, Y, Zr, W and the lanthanides.
- Lithophile elements mainly consist of the highly reactive metals of the s- and f-blocks.
- They also include a small number of reactive nonmetals, and the more reactive metals of the d-block such as titanium, zirconium and vanadium.
- Lithophile derives from "lithos" which means "rock", and "phile" which means "love".
- Most lithophile elements form very stable ions with an electron configuration of a noble gas.
- The few that do not, such as silicon, phosphorus and boron, form extremely strong covalent bonds with oxygen – often involving bonding.
2. Sidrophile Elements
- Siderophile (from sideron, "iron", and philia, "love") elements are the transition metals which tend to sink into the core because they dissolve readily in iron either as solid solutions or in the molten state, although some sources include elements which are not transition metals in their list of siderophiles, such as germanium.
- Other sources may also differ in their list based on the temperature being discussed - niobium, vanadium, chromium, and manganese may be considered siderophiles or not, depending on the assumed temperature and pressure.
- Also confusing the issue is that some elements, such as the manganese, as well as molybdenum, form strong bonds with oxygen, but in the free state (as they existed on the primitive Earth when free oxygen did not exist) can mix so easily with iron that they do not concentrate in the siliceous crust, as do true lithophile elements.
- The siderophile elements include the highly siderophilic ruthenium, rhodium, palladium, rhenium, osmium, iridium, platinum, and gold, the moderately siderophilic cobalt and nickel, in addition to the "disputed" elements mentioned earlier - some sources even include tungsten and silver.
3. Chalcophile Elements
- The chacophile elements include: Ag, As, Bi, Cd, Cu, Ga, Ge, Hg, In, Pb, S, Sb, Se, Sn, Te, Tl and Zn.
- Chalcophile elements are those that remain on or close to the surface because they combine readily with sulfur and/or some other chalcogen other than oxygen, forming compounds which do not sink into the core.
- Chalcophile elements are those metals and heavier nonmetals that have a low affinity for oxygen and prefer to bond with sulfur as highly insoluble sulfides.
- Chalcophile derives from Greek khalkós , meaning "ore" (it also meant "bronze" or "copper", but in this case "ore" is the relevant meaning), and is taken to mean "chalcogen-loving" by various sources.
4. Atmophile Elements
- The atmophile elements are H, C, N and the noble gases.
- Atmophile elements (also called "volatile elements") are defined as those that remain mostly on or above the surface because they are, or occur in, liquids and/or gases at temperatures and pressures found on the surface.
- The noble gases do not form stable compounds and occur as monatomic gases, while nitrogen, although it does not have a stable configuration for its individual atoms, forms a diatomic molecule so strong that all oxides of nitrogen are thermodynamically unstable with respect to nitrogen and oxygen.



