Week 4-5. Mobility and dispersion of elements under different geochemical environments
Mobility and dispersion of elements under different geochemical environments
The distribution of elements of the constituents rocks and minerals in response to various geological process are referred to as geochemical mobility, while the movement of elements below the Earth's surface by metamorphic, magmatic, or hydrothermal processes is called geochemical dispersion.
Introduction:
So far we have viewed the concentrations of species in the atmosphere as controlled by emissions, transport, chemistry, and deposition. From an Earth system perspective, however, the composition of the atmosphere is ultimately controlled by the exchange of elements between the different reservoirs of the Earth. Atmospheric composition from this broader perspective, and focus more specifically on the biogeochemical factors that regulate the atmospheric abundances of N2, O2, and CO2.
The Earth system (including the Earth and its atmosphere) is an assemblage of atoms of the 92 natural elements. Almost all of these atoms have been present in the Earth system since the formation of the Earth 4.5 billion years ago by gravitational accretion of a cloud of gases and dust. Subsequent inputs of material from extraterrestrial sources such as meteorites have been relatively unimportant. Escape of atoms to outer space is prevented by gravity except for the lightest atoms (H, He), and even for those it is extremely slow . Thus the assemblage of atoms composing the Earth system has been roughly conserved since the origin of the Earth. The atoms, in the form of various molecules, migrate continually between the different reservoirs of the Earth system. Geochemical cycling refers to the flow of elements through the Earth's reservoirs; the term underlines the cyclical nature of the flow in a closed system.
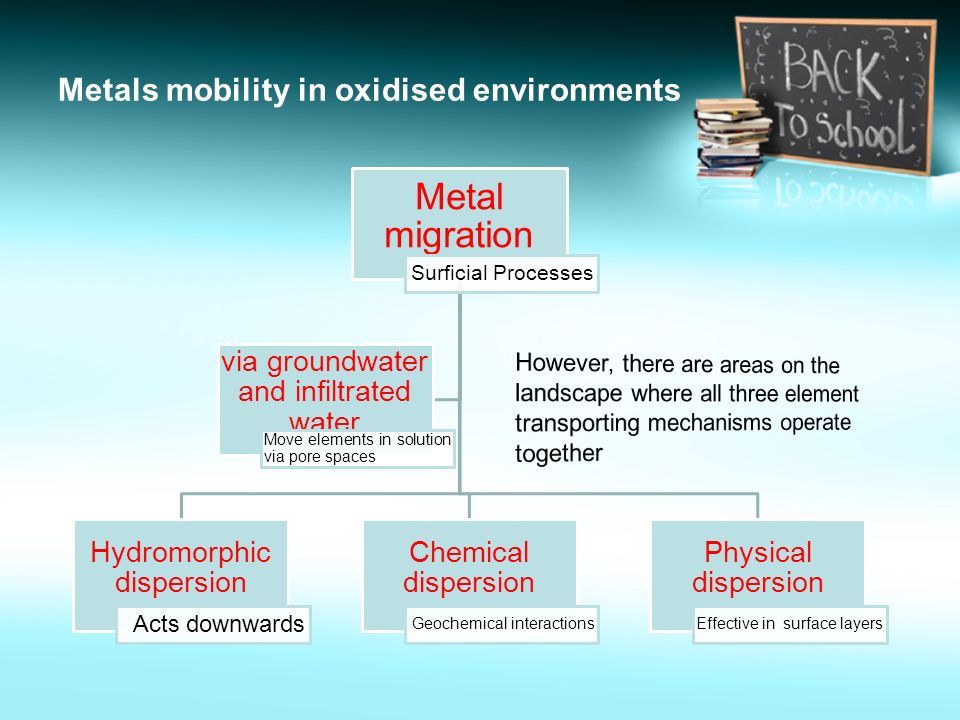
THE CHEMICAL MOBILITY AND TRANSPORT OF ELEMENTS IN THE WEATHERING ENVIRONMENT
The aqueous solution, transport and precipitation of elements is of central importance to weathering and geochemical dispersion. The hydrolysis and redox reactions are principally those that affect the major elements, being responsible for the destruction of the primary rock-forming minerals and the formation of the secondary minerals of the regolith. Of particular importance to geochemical exploration, however, are those reactions that affect the generally less abundant elements associated with mineralization. These are the reactions that are ultimately responsible for the nature of the surface expression of mineralization in the regolith, particularly its size, intensity and location. Geochemical anomalies formed as the result of chemical reactions and transport involving water are referred to as hydromorphic and the process as hydromorphic dispersion. The purpose of this chapter is to describe the chemical principles involved in hydromorphic dispersion and the manner in which individual elements or groups of elements behave in different weathering environments. The extent to which particular elements are mobilized by groundwaters during weathering depends upon:
(1) the controls of solubility in aqueous solutions having various amounts of other dissolved species (cations, anions and organic materials),
(2) the interaction between these solutions and the complex surfaces of primary and secondary minerals.
Interactions between the two phases take place at every point on the interface. The various elements may be: (a) held within the mineral crystal lattice; (b) loosely bound within a layer of the changing potential field at the interface; or (c) mobile in solution, either solvated by the water molecules or "complexed" with one or more other soluble ions. Mobility is also possible as colloidal particles; these particles will have a similar dynamic interface with the solution.



