WEEK 5:TRANSPORT ACROSS MEMBRANE
Diffusion:
All molecules and ions in the body fluids, including
water molecules and dissolved substances, are in constant
motion, each particle moving its own separate
way. Motion of these particles is what physicists call
“heat”—the greater the motion, the higher the temperature—
and the motion never ceases under any
condition except at absolute zero temperature.When
a moving molecule, A, approaches a stationary molecule,
B, the electrostatic and other nuclear forces of
molecule A repel molecule B, transferring some of the
energy of motion of molecule A to molecule B. Consequently,
molecule B gains kinetic energy of motion,
while molecule A slows down, losing some of its
kinetic energy. Thus, a single
molecule in a solution bounces among the other
molecules first in one direction, then another, then
another, and so forth, randomly bouncing thousands of
times each second. This continual movement of molecules
among one another in liquids or in gases is called
diffusion.
Ions diffuse in the same manner as whole molecules,
and even suspended colloid particles diffuse in a
similar manner, except that the colloids diffuse far
less rapidly than molecular substances because of their
large size.
Facilitated Diffusion:
Facilitated diffusion is also called carrier-mediated diffusion
because a substance transported in this manner
diffuses through the membrane using a specific carrier
protein to help. That is, the carrier facilitates diffusion
of the substance to the other side.
Facilitated diffusion differs from simple diffusion in
the following important way: Although the rate of
simple diffusion through an open channel increases
proportionately with the concentration of the diffusing
substance, in facilitated diffusion the rate of
diffusion approaches a maximum, called Vmax, as the
concentration of the diffusing substance increases. As the concentration of the diffusing substance
increases, the rate of simple diffusion continues to
increase proportionately, but in the case of facilitated
diffusion, the rate of diffusion cannot rise greater than
the Vmax level.
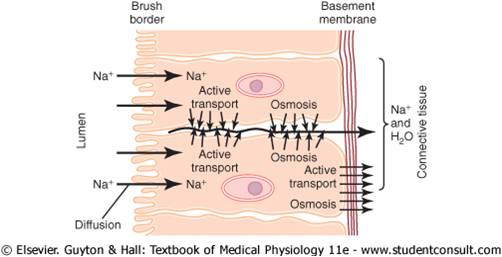
“Active Transport” of
Substances Through
Membranes:
At times, a large concentration of a substance is
required in the intracellular fluid even though the
extracellular fluid contains only a small concentration.
This is true, for instance, for potassium ions. Conversely,
it is important to keep the concentrations of
other ions very low inside the cell even though their
concentrations in the extracellular fluid are great.This
is especially true for sodium ions. Neither of these two
effects could occur by simple diffusion, because simple
diffusion eventually equilibrates concentrations on the
two sides of the membrane. Instead, some energy
source must cause excess movement of potassium ions
to the inside of cells and excess movement of sodium
ions to the outside of cells. When a cell membrane
moves molecules or ions “uphill” against a concentration
gradient (or “uphill” against an electrical or pressure
gradient), the process is called active transport.
Different substances that are actively transported
through at least some cell membranes include sodium
ions, potassium ions, calcium ions, iron ions, hydrogen
ions, chloride ions, iodide ions, urate ions, several different
sugars, and most of the amino acids.
Primary Active Transport and Secondary Active Transport.:
Active transport is divided into two types according to
the source of the energy used to cause the transport:
primary active transport and secondary active transport.
In primary active transport, the energy is derived
directly from breakdown of adenosine triphosphate
(ATP) or of some other high-energy phosphate compound.
In secondary active transport, the energy is
derived secondarily from energy that has been stored
in the form of ionic concentration differences of
secondary molecular or ionic substances between the
two sides of a cell membrane, created originally by
primary active transport. In both instances, transport
depends on carrier proteins that penetrate through the
cell membrane, as is true for facilitated diffusion.
However, in active transport, the carrier protein functions
differently from the carrier in facilitated diffusion
because it is capable of imparting energy to the
transported substance to move it against the electrochemical
gradient. Following are some examples
of primary active transport and secondary active
transport, with more detailed explanations of their
principles of function.


