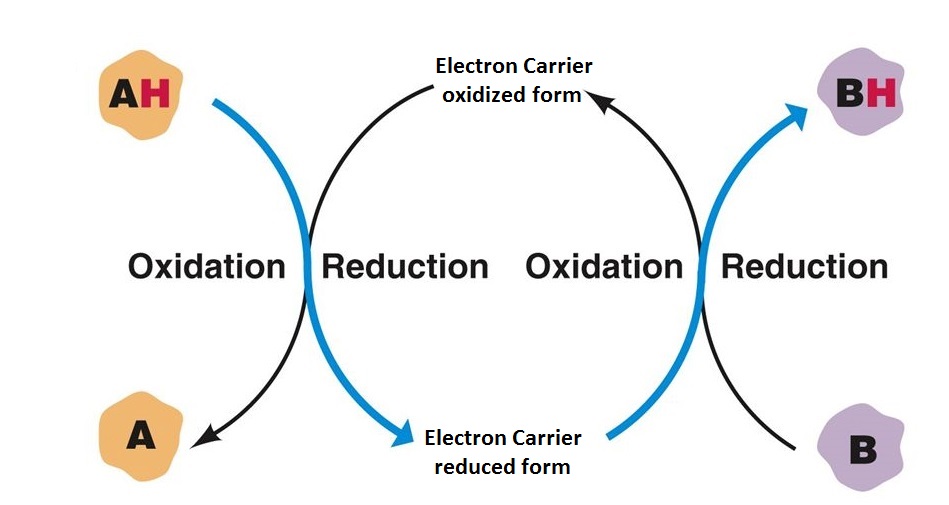
REDOX Reactions & Reduction Potentials
- The electron lost in the oxidation is accepted by an acceptor which is said to be reduced. • Commonly oxidation reactions are accompanied by reduction reactions, and they are called as Redox Reactions.
- High redox potential signifies High Electron Affinity. • Low redox potential signifies Low Electron Affinity. • More negative ( or low) redox potential Greater Tendency to lose Electrons • More positive ( or high) redox potential Greater Tendency to accept Electrons


