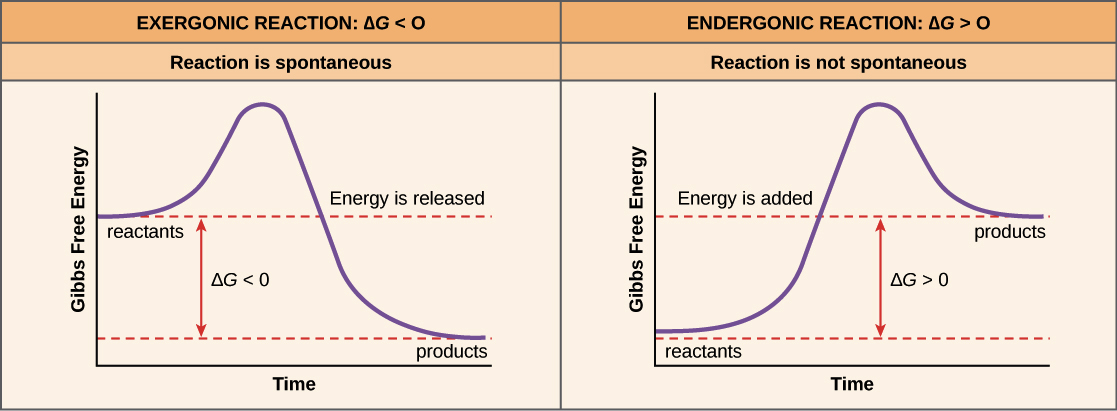
EXERGONIC & ENDERGONIC REACTIONS AND COUPLING
All reactions in biological systems are considered to be reversible reactions, so that the free energy of the reverse reaction numerically equivalent, but opposite in sign to that of the forward reaction.
Exergonic reactions result in products with less energy than the reactants.
Endergonic reactions result in products with more energy than the reactants.
Endergonic reactions are proceeded by coupling to Exergonic reactions for energy requirements.


