Lipids (Properties)
Physical properties
The physical properties of the fatty acids, and of compounds that contain them, are largely determined by the length and degree of unsaturation of the hydrocarbon chain. The nonpolar hydrocarbon chain accounts for the poor solubility of fatty acids in water. For example, Lauric acid (12:0, M mass 200) has a solubility in water of 0.063 mg/g much less than that of glucose (M mass 180), which is 1,100 mg/g. The longer the fatty acyl chain and the fewer the double bonds, the lower is the
solubility in water.
➢ The carboxylic acid group is polar (and ionized at neutral pH) and accounts for the slight solubility of shortchain fatty acids in water.
➢ Melting points are also strongly influenced by the length and degree of unsaturation of the hydrocarbon chain.
➢ At room temperature (25 °C), the saturated fatty acids from 12:0 to 24:0 have a waxy consistency, whereas unsaturated fatty acids of these lengths are oily liquids.
➢ This difference in melting points is due to different degrees of packing of the fatty acid molecules. In the fully saturated compounds, free rotation around each carbon–carbon bond gives the hydrocarbon chain great flexibility; the most stable conformation is the fully extended form, in which the steric hindrance of neighboring atoms is minimized.
➢ These molecules can pack together tightly in nearly crystalline arrays, with atoms all along their lengths in van der Waals contact with the atoms of neighboring molecules.
➢ In unsaturated fatty acids, a cis double bond forces a kink in the hydrocarbon chain. Fatty acids with one or several such kinks cannot pack together as tightly
as fully saturated fatty acids, and their interactions with each other are therefore weaker.
➢ Because less thermal energy is needed to disorder thesepoorly ordered arrays of unsaturated fatty acids, they have markedly lower melting points than saturated fatty acids of the same chain length
➢ poorly ordered arrays of unsaturated fatty acids, they have markedly lower melting points than saturated fatty acids of the same chain length
➢ In vertebrates, free fatty acids (unesterified fatty acids, with a free carboxylate group) circulate in the blood bound no covalently to a protein carrier, serum albumin.
➢ However, fatty acids are present in blood plasmamostly as carboxylic acid derivatives such as esters or amides. Lacking the charged carboxylate group, these fatty acid derivatives are generally even less soluble in water than are the free fatty acids.
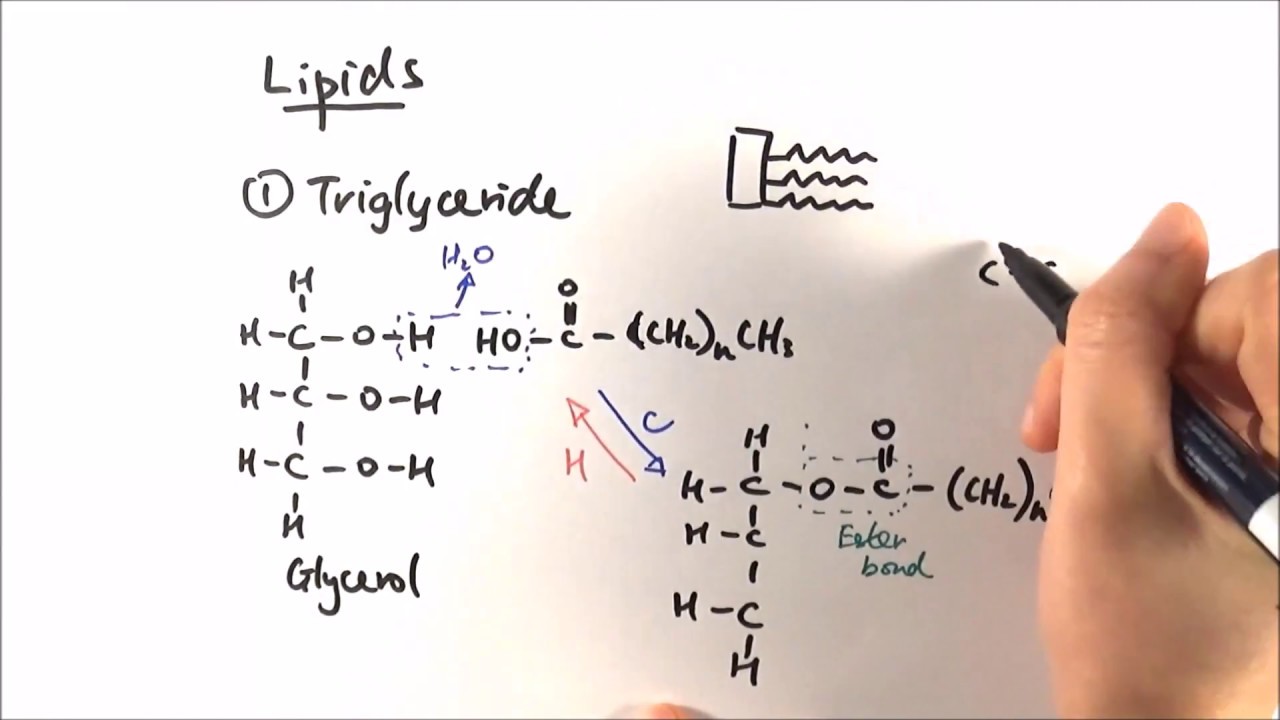
Triacylglycerides Are Fatty Acid Esters of Glycerol
The simplest lipids constructed from fatty acids are the triglycerides, also referred to as triacylglycerols, fats, or neutral fats. Triacylglycerols are composed of three fatty acids, each in ester linkage with a single glycerol (Fig. 10-2). Those containing the same kind of fatty acid in all three positions are called simple triacylglycerols and are named after the fatty acid they contain. Simple triacylglycerols of 16:0, 18:0, and 18:1, for example, are tripalmitin, tristearin, and triolein, respectively. Most naturally occurring triacylglycerols are mixed; they contain two or three different fatty acids. To name these compounds unambiguously, the name and position of each fatty acid must be specified.
Because the polar hydroxyls of glycerol and the polar carboxylates of the fatty acids are bound in ester linkages, triacylglycerols are nonpolar, hydrophobic molecules, essentially insoluble in water. Lipids have lower specific gravities than water, which explains why mixtures of oil and water (oil-and-vinegar salad dressing, for example) have two phases: oil, with the lower specific gravity, floats on the aqueous phase.

Structural Lipids in Membranes
1. The central architectural feature of biological membranes is a double layer of lipids, which acts as a barrier to the passage of polar molecules and ions.
2. Membrane lipids are amphipathic: one end of the molecule is hydrophobic, the other hydrophilic. Their hydrophobic interactions with each other and their
hydrophilic interactions with water direct their packing into sheets called membrane bilayers.
Five general types of membrane lipids:
1. Glycerophospholipids; in which the hydrophobic regions are composed of two fatty acids joined to glycerol;
2. .Galactolipids and sulfolipids; which also contain two fatty acids esterified to glycerol, but lack the characteristic phosphate of phospholipids;
3. Archaeal tetraether lipid; in which two very long alkyl chains are ether-linked to glycerol at both ends;
4. Sphingolipids; in which a single fatty acid is joined to a fatty amine.
5. Sphingosine; and sterols, compounds characterized by a rigid system of four fused hydrocarbon rings. The hydrophilic moieties in these amphipathic compounds
may be as simple as a single —OH group at one end of the sterol ring system, or they may be much more complex.
6. In glycerophospholipids and some sphingolipids, a polar head group is joined to the hydrophobic moiety by a phosphodiester linkage; these are the
phospholipids.
7. Glycolipids; Other sphingolipids lack phosphate but have a simple sugar or complex oligosaccharide at their polar ends; these are the glycolipids. Within these groups of membrane lipids, enormous diversity results from various combinations of fatty acid “tails” and polar “heads.” The arrangement of these lipids in membranes, and their structural and functional roles.


