Acid-Base Regulation
Regulation of hydrogen ion (H+) balance is similar in some ways to regulation of other ions in the body. For instance, there must be a balance between the intake or production of H+ and net removal of H+ from the body to achieve homeostasis. And, as is true for other ions, the kidneys play a key role in regulating H+ removal from the body. However, precise control of extracellular fluid H+ concentration involves much more than simple elimination of H+ by the kidneys. Multiple acid-base buffering mechanisms involving the blood, cells, and lungs also are essential in maintaining normal H+ concentrations in both the extracellular and intracellular fluid. Here, we consider the various mechanisms that contribute to the regulation of H+ concentration, with special emphasis on control of renal H+ secretion and renal reabsorption, production, and excretion of bicarbonate ions (HCO3 −), one of the key components of acidbase control systems in the body fluids.
A balance between input and output of H+ is critical to maintaining the body’s acid–base balance within the narrow limits compatible with life. Deviations in the internal fluid environment’s pH lead to altered neuromuscular excitability, to changes in enzymatically controlled metabolic activity, and to K1 imbalances, which can cause cardiac arrhythmias. These effects are fatal if the pH falls outside the range of 6.8 to 8.0. Hydrogen ions are uncontrollably and continuously added to the body fluids as a result of ongoing metabolic activities, yet the ECF pH must be kept constant at a slightly alkaline level of 7.4 for optimal body function. Like salt and H2O balance, control of H+ output by the kidneys is the main regulatory factor in achieving H+ balance. The lungs, which can adjust their rate of excretion of H+-generating CO2, also help eliminate H+ from the body. Furthermore, chemical buffer systems can take up or liberate H+, transiently keeping its concentration constant within the body until its output can be brought into line with its input. Such a mechanism is not available for salt or H2O balance.
At the end of the lesson, students will be able to understand;
H+ CONCENTRATION REGULATION
BUFFERING OF H+ IN THE BODY FLUIDS
RESPIRATORY REGULATION OF ACID-BASE BALANCE
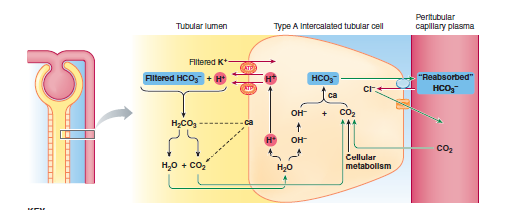
RENAL CONTROL OF ACID-BASE BALANCE
QUANTIFYING RENAL ACID-BASE EXCRETION
RENAL CORRECTION OF ACIDOSIS AND ALKALOSIS


